What is a Good Manufacturing Practices (GMP) Inspection Checklist?
Certainty Software’s Good Manufacturing Practices (GMP) Inspection Checklist measures a facility or organization’s compliance with the FDA’s Good Manufacturing Practices (GMP) – Active Pharmaceutical Ingredients. During inspections, your team will be able to more quickly and effectively identify the root causes of nonconformances and develop corrective actions to meet regulatory standards.

Download the Good Manufacturing Practices (GMP) Inspection Checklist
What the Checklist Includes
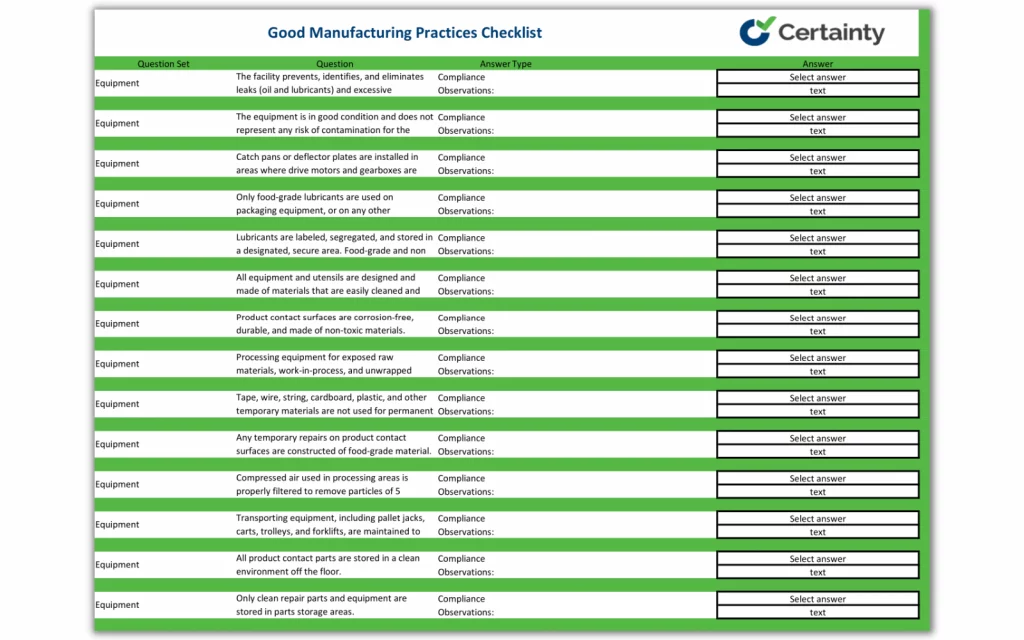
This 115-question checklist details the many aspects of GMP regulations compliance necessary for overall food safety success and quality assurance. The checklist includes aspects of the following:
- Equipment cleaning
- Testing procedures
- Storage areas
- Cross-contamination
- Pesticides
- Hand washing
- Food contact
- Standing water
- Freezers
- Warehousing
- Written procedures
- Toilet facilities
- Labeling
- Pest control
- Raw materials
- and more.
How Certainty Improves Good Manufacturing Practices (GMP) Inspections
Using Certainty Software, performing GMP inspections becomes easier and generates better actionable information. Whether using our checklist templates or creating your own unique forms, Certainty gives its users the freedom and customizability to support every company’s unique inspection and auditing needs.
Reporting checklist findings at an enterprise-wide level can be filtered to your needs by options such as inspection type, users, site, region, question, or answer. Within your configurable dashboard, you can track issues identified, set up automated notifications and actions, and so much more.
For more on GMP Inspection solutions, click here.

Spend Time On Prevention
Not Paperwork
Watch our overview video to see how your organization can benefit from Certainty.
Watch Video



